USP/IP Compliance Tablet Dissolution tester (Dissolution test Apparatus) Manufacturer India
Dissolution tester for Medicine Tablet/12 station Tablet Dissolution Test Apparatus / 14 Vessel Tablet Dissolution Tester Apparatus/ Capsule dissolution testing in Dissolution Test apparatus

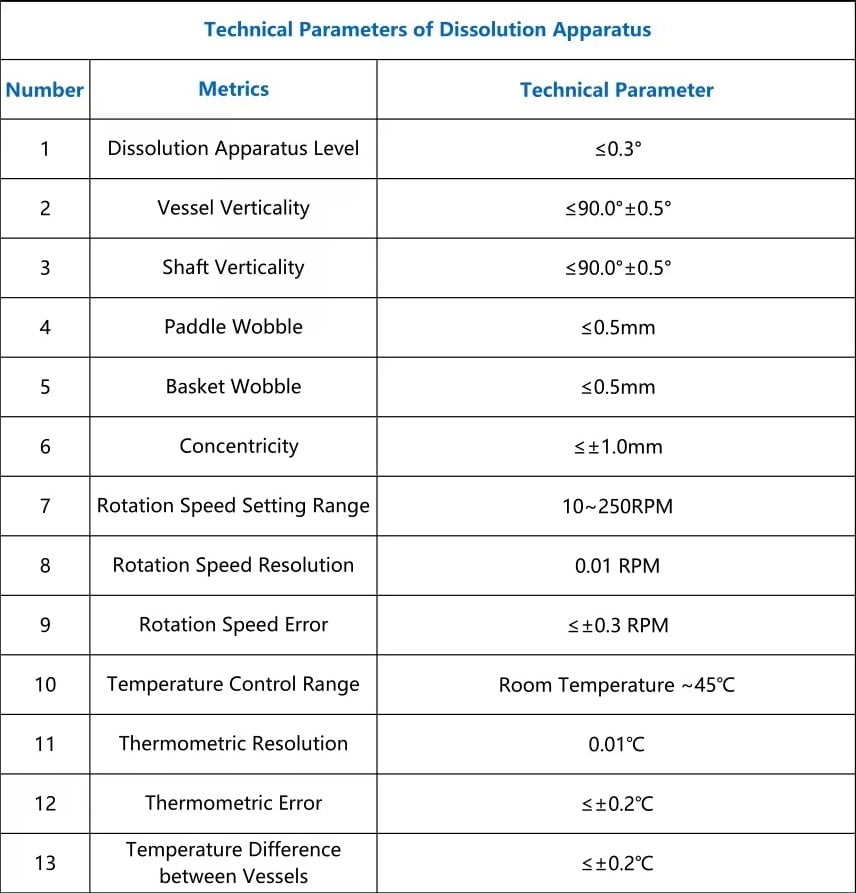
Dissolution tester is designed to determine the rate at which tablets and capsules completely dissolve into the gastrointestinal tract and releases drug ingredients in the body. This instrument evaluates the bio-availability of the tablet or capsule and provides accurate information to the team. Our instrument is used in the quality control & R&D laboratory of pharmaceuticals for testing the USP/IP compliance in the making of a drug.
This Instrument comes with the latest technology, Microprocessor controlled; advanced engineering techniques, 4 lines alphanumeric LCD display with 19 soft touch keys, 200 sample storage facility & ISI mark SS heater for uniform temperature. This instrument has a facility for setting up 20 different kinds of test methods.
Yatherm microprocessor-based test apparatus comes with an acrylic water bath, SS heaters & programmable stirrers for accurate and best results. This instrument works on standard temperature & RPM by keeping drugs into the reaction vessel for a standard time and checks the dissolution of the drug. SS heaters give uniform temperature to the water bath and drugs while running this test. This test is very useful for pharmaceutical companies to get FDA approval from the government.
The Yatherm Dissolution test apparatus comes in different stations for different types of users with user-friendly features. We manufacture and offer high-quality equipment with the best price in the WORLD for our customers. We offer a year warranty with five years of spare parts availability to all our customers at the best price in India.

Features
- 1, 6, 8 & 14 station models are available
- USP/IP compliance
- Accurate and precise testing
- 20-200 RPM programmable speed
- Three programming modes
- 19 soft touch key for higher models
- Samples storage facility after machine off
- Easy to clean the water bath and beakers
- Printer port
- Audio-visual alarm system

Specifications
| DISSO – X series | |
| Stations | 1, 6, 8 & 14 station models are available |
| Display | 20 x 4 Line Alphanumeric LCD display with Backlit |
| Keyboard | 19 soft-touch membrane keys |
| Prog. Mode | Routine, Sustain and Control mode |
| Printer | Provision for the attachment of a real-time dot-matrix printer |
| Stirrer | |
| Speed | 25 – 200 RPM, ± 1 RPM |
| Resolution | 1 RPM |
| Accuracy | ± 1 RPM |
| Temperature | |
| Range | Room temperature – 100°C |
| Accuracy | +/- 1°C |
| Temp. Control | Microprocessor Based using PT 100 |
| Sampling | |
| Reaction Vessel | 1000 ml jars, stirrer pedals, and baskets |
| Heater | 500 watt to 1000 kW as per model |
| Power | Single-phase 220 v |
| Accessories (inclusive) | Water Bath, Temperature Sensor, External Temperature Sensor, Reaction Vessel capacity 1000 ml, Round Acrylic Cover Plate, S.S. Paddle, S.S Wire Mesh Basket |
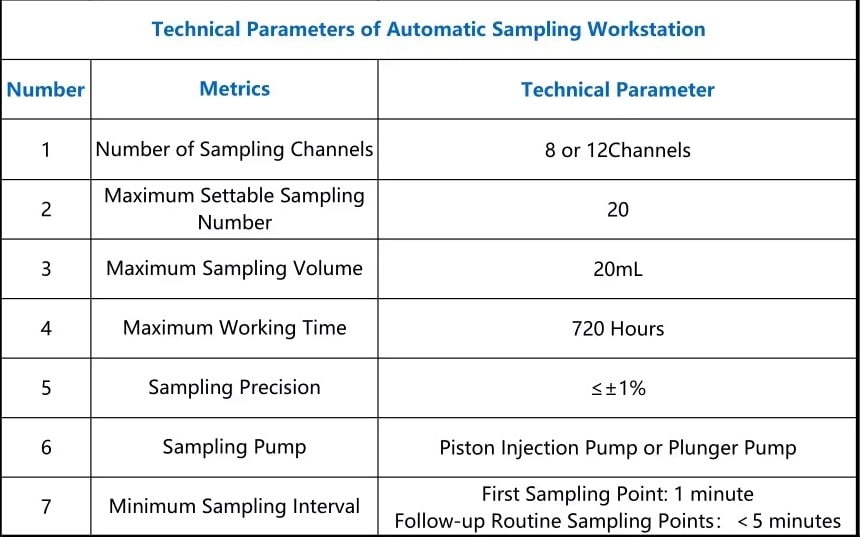
Dissolution test Apparatus with Automatic Sampling Work-Station.
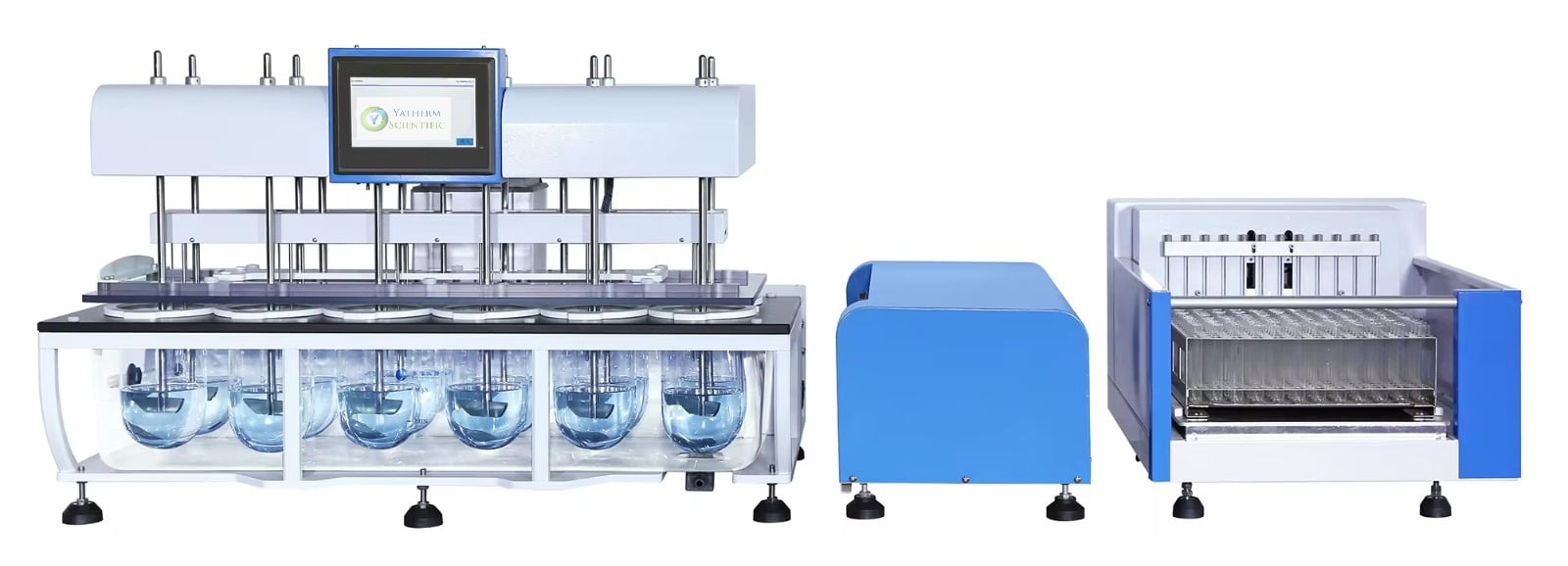
Yatherm Dissolution Test Apparatus is designed and manufactured in accordance with the Indian Pharmacopoeia and the US Pharmacopoeia. The Working principle of Dissolution tester for mechanical dissolution of drugs follows the International standards. This is our latest model with high performance with 12 jars & 12 rod dissolution tester.
Features of Drug Dissolution Tester
- Testers are designed as per “Indian Pharmacopoeia” and “United States Pharmacopoeia”
- Follows the guidelines of Validation of Dissolution Test Apparatus
- Equipped with 12 Jars with 12 Rods
- Driving head has intelligent automatic lifting
- Adjustment of height
- Center position setting of the stirrer
- Accurate Jar Centering
- High quality temperature probe to monitor the water bath temperature
- Accurate Speed motor
- Color Touch screen HMI
- Fully complies GMP & other international regulations