High Stability Pharmaceutical Stability Chambers/ Comprehensive Drug Test Chamber Manufacturer India/ Drug Stability Test chamber – single door/drug Stability/ Medicine ICH Stability Climatic Chamber/ Pharmaceutical Stability Test Chambers/Stability Testing Chamber lab research Stability Testing Chamber in controlled environment/Drug Stability Test Chamber

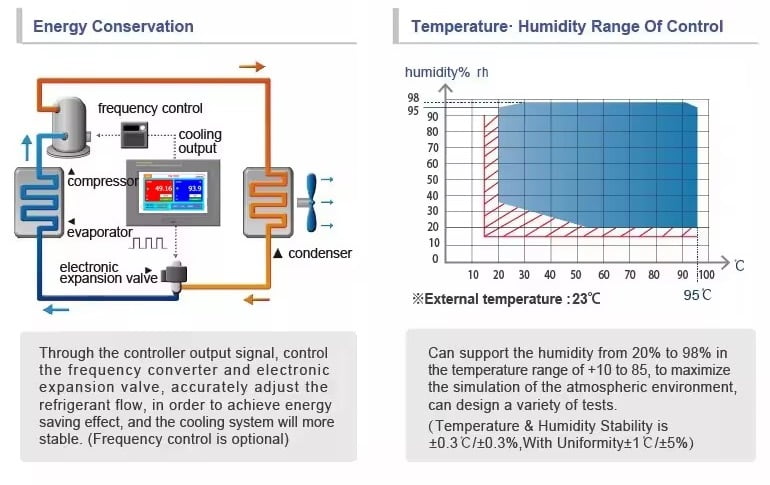
Yatherm Stability test Chamber fulfills the advanced specifications for pharmaceutical, food & defense laboratories. Modern & latest designs of cabinet equipped with advance HCFC free refrigeration system, PLC operated temperature controlling device with Rust-free humidity Reservoir. We offer thick PUF insulation for energy conservation with a safe environment for the user. Stability test chambers are designed for continuous operation under the observation of scientists for up to 90 days without any interruption.
Pharmaceutical industry follows chemical & drug stability guidelines to perform test – drug production and development shall be specified temperature, humidity and light irradiation test. These tests are divided into 4 to 5 categories and these categories mentioned below with standard temperature, Humidity and light requirement.
High humidity test: Temperature of 25 ℃ ± 2 ℃, humidity 90% R.H ± 5%, or 75% ± 5%. Generally high humidity tests are performed between 85% to 95% relative humidity at ambient temperature.
Acceleration test: 40 ℃ ± 2 ℃, 75% ± 5% or 30 ℃ ± 2 ℃, 65% ± 5% .; Packaging in pharma industry formulations for semi-permeable containers, like plastic sealed boxes, PVC bottle drops liquid, Nasal drops should be 40 ℃ ± 2 ℃, 20% ± 5% or 25 ℃ ± 2 ℃, 20% ± 5%. This test helps in analyzing the impact of climate and transportation on drug formulation
Long-term test: Ambient temperature 25 ℃ ± 2 ℃, Relative Humidity 60% ± 10%; in pharma industry formulations for packing in leakproof Plastic containers, such as PVC bags solution, PVC bottles for eye drops & nasal drops should be 25 ℃ ± 2 ℃, 40% ± 10%.
Additional tests in moderate conditions: 30℃±2℃, 40%±5%
High temperature test: 40 ℃, 60 ℃
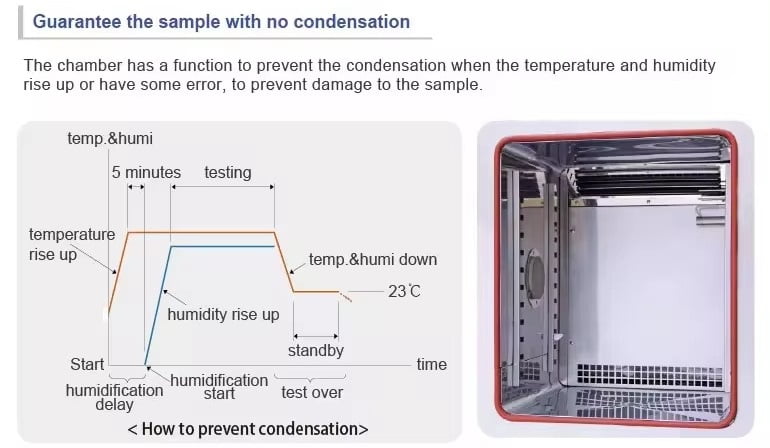
Stability test chambers are different from the temperature Humidity test chamber in terms of long-term Storage Testing with precise and accurate controlling. Stability test chambers are highly accurate & stable to maintain the standard environment for pharmaceutical drugs. Yatherm Stability test chambers come with a safe & sound performance for temperature utilization inside the chamber. ICH Stability test chambers have a wide working range that meets the requirements of the International standard as per ICH guidelines Q1A (R2) for stability testing.
Fog-free viewing window and interior light make viewing workspace freely and observe the test under the best conditions. The left side of the chamber with a diameter 50 mm (support customized) cable port for the power-on test. The optional electronic humidity sensor is used on all test chambers for accuracy and minimal maintenance. Safety relay connection is provided to protect your device under test by removing power to it when the chamber is not running. The touch screen controller is designed to save chamber programming and setup time with temperature limits an alarm to protect your product.
Standard Features
- PLC with 3.5 to 7 inches HMI for temperature, Light and humidity controlling
- Temperature testing Range from +10°C to +50°C for standard Models
- Humidity range from 40%RH to 95%RH
- Adjustable perforated SS 304 trays
- GAMP 4 compliance
- Capacitive humidity sensor
- SS 304/ SS 316 Chamber
- Energy Saver up to 60%
- Extremely low sound pressure level
- ISO/CE/GMP certification
- Adjustable product shelf slides out for easier product access
- Shelf design is non-tipping and supports large product loads
Chamber Construction
- Chamber is designed with SS 304 grade Steel body
- Easy to clean & maintain
- High Quality Air duct for proper air ventilation
- Perforated SS 304/SS 316 grade trays
You Demand and Yatherm design : Stability Test chamber are specially designed for long working hours accelerated testing & evaluation of drugs failure. Pharmaceutical industry performs accelerated test on life science projects, Microbiology projects, biotechnology labs project & biological applications.
Technical Specification:
|
YATHERM MODEL |
BENZO -120 |
BENZO – 225 |
BENZO – 400 |
BENZO – 500 |
BENZO – 700 |
|
Studio size |
500x450x600 |
500x500x900 |
660x550x1100 |
750x600x1100 |
900x700x1100 |
|
Dimensions |
980x630x1320 |
980x680x1600 |
1100x730x1700 |
1200x780x1700 |
1000x1400x1700 |
|
Install the power |
1.0Kw |
1.2 Kw |
1.5 Kw |
1.5 Kw |
1.5 Kw |
|
Number of shelves |
2 |
3 |
4 |
4 |
4 |
|
Door size |
750mm |
800mm |
750mm |
||
|
Temperature range |
+10–65℃ |
||||
|
Humidity range |
40-95%R.H |
||||
|
Temperature fluctuation |
<±0.5℃ |
||||
|
Temperature uniformity |
<2.0℃ |
||||
|
Temperature deviation |
<±1.0℃ |
||||
|
Humidity deviation |
<±3.0%R.H |
||||
|
Temperature and humidity controller |
|
||||
|
Sensor |
|
||||
|
Data record |
|
||||
|
Heating system |
Nickel-chromium alloy electric heater |
||||
|
Humidification system |
Special electric steam generator – Injecting type |
||||
|
Cooling System |
|
||||
|
Dehumidification system |
Using a dehumidifier, dehumidifier can be adjusted |
||||
|
The door sealed |
|
||||
|
Door handle |
Buckle-type locking device with a door handle , Data Logging for door entry. |
||||
|
Water system |
|
||||
|
Wireless alarm |
|
||||
|
Safety protection |
|
||||
|
Standard configuration |
|
||||
|
Optional |
Recorder & network interface |
||||
|
Product Standards |
Test Chamber meets the ICH2003 Q1A (R2) |
||||
|
Power supply |
220V ± 10%, 50Hz, |
||||
|
Liner material |
SS304 mirror stainless steel |
||||
|
21 CFR Controller Compliance |
|
||||
Note: We manufacture customized chamber with customized specifications in terms of Humidity, temperature & Dimensions.
What is a Pharmaceutical Stability Test Chamber ?
Pharma Stability Test Chambers from Yatherm Scientific are specially designed for the high end requirements in shelf life testing, expiry date testing, climatic change testing, accelerated aging testing & stability studies. Stability test chambers should follow the ICH stability guidelines for controlled and uniformity of temperature and relative humidity.
We manufacture and produce reliable stability test chambers in powerful design for API, pharmaceutical and Drug formulation industries in product research & development. We manufacture 200 Liters, 450 Liters , 750 Liters & 1500 Liters with walk in size of 3000 Liters. We can customize Dimensions according to the specimen size and parameters according to the testing requirements.
- Drug stability chambers are applied to pharmaceutical industries for the evaluation of drug failure, Pharma GMP certification, pharma stability testing & accelerated testing and Long duration trails.
- Test Holes / Cable Hole – Internal Diameters of 50 mm, 125 mm or custom size diameter with rubber sealing & SS 304 cover.

- Large Viewing Window size of 250 x 300 mm for the observation of humidity & temperature on sample. Window is protected from temperature and humidity.
- Drug Stability Test chambers are designed According to INDIAN Pharmacopoeia, US pharmacopoeia, AS3100 of Australia, ICH & other International standards.
- All kind of validation documents IQ, OQ, PQ with related certificates like Calibration reports.
- CE & GMP certified with three years warranty.
Quotation Request Form


